PAYPAL: Clicca qui
STRIPE: Clicca qui
In alternativa, è possibile effettuare un bonifico bancario (SEPA) utilizzando il nostro conto
Titolare del conto: Come Don Chisciotte
IBAN: BE41 9674 3446 7410
BIC: TRWIBEB1XXX
Causale: Raccolta fondi
LONDON — The U.K.’s Medicines & Healthcare products Regulatory Agency (MHRA) is the functional equivalent of the Food and Drug Administration (FDA) in the USA. MHRA granted emergency use authorization for the Pfizer BioNTech mRNA shots last December. It also approved the Oxford-AstraZeneca viral vector vaccine and Moderna mRNA shots for emergency use.
The Centers for Disease Control manages the Vaccine Adverse Event Reporting System (VAERS) in the United States. It allows healthcare providers and any other American to report adverse reactions to mRNA shots and other vaccines. The Yellow Card scheme, managed by MHRA, is the equivalent reporting database in the U.K.
MHRA published a report on February 11 summarizing the self-reported adverse effects to Pfizer BioNTech and Oxford-Astra Zeneca shots from December 9, 2020 to January 31, 2021. The usual suspects, like anaphylaxis (120), Bell’s Palsy (99) and death (66), show up in the report. But some new adverse reactions have surfaced that even this blogger has not seen until now.
Quintuple blind experiment
MHRA defines temporal association as “events occurring following vaccination but may or may not be caused by the vaccine.” It is indisputable, however, that the risks far outweigh the social acceptance and temporary social media fame gained from being injected with mRNA.
Twelve people reported going deaf after receiving the Pfizer BioNTech shots.
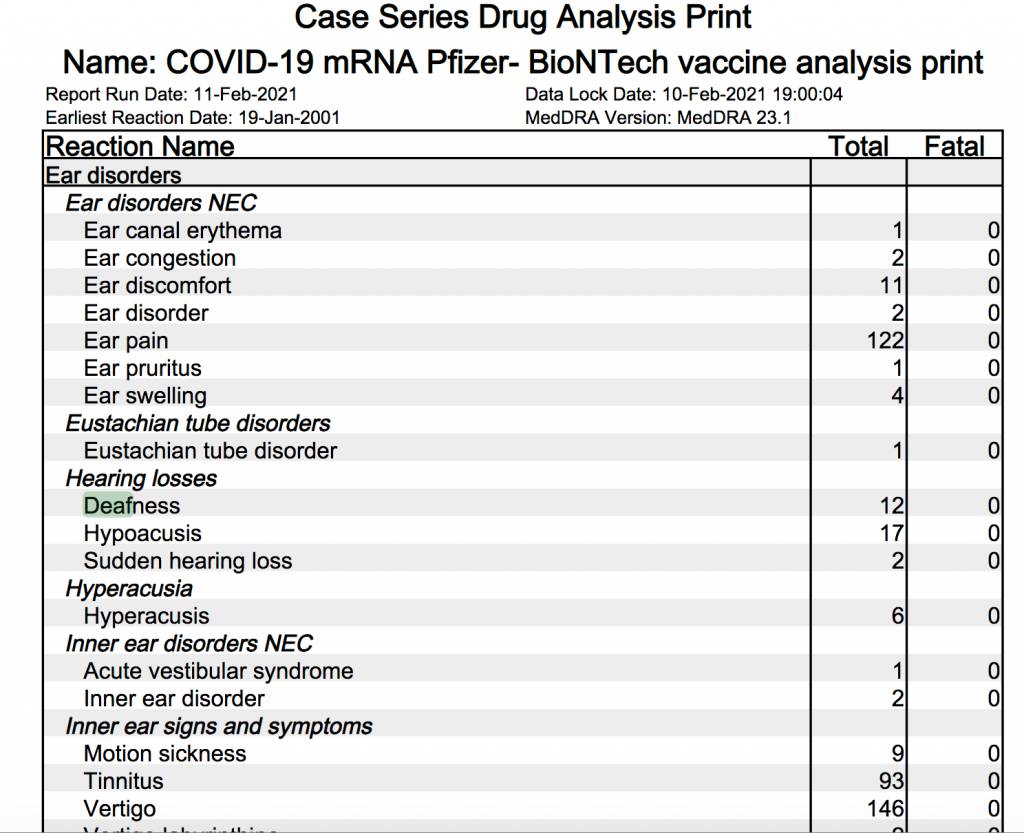 Meanwhile five people reported going blind after receiving the mRNA shot.
Meanwhile five people reported going blind after receiving the mRNA shot.
 You can view the full report here.
You can view the full report here.
Fonte articolo: https://thecovidblog.com/2021/02/16/united-kingdom-12-deaf-five-blind-after-pfizer-mrna-shots/
CANALE YOUTUBE: https://www.youtube.com/@ComeDonChisciotte2003
CANALE RUMBLE: https://rumble.com/user/comedonchisciotte
CANALE ODYSEE: https://odysee.com/@ComeDonChisciotte2003
CANALI UFFICIALI TELEGRAM:
Principale - https://t.me/comedonchisciotteorg
Notizie - https://t.me/comedonchisciotte_notizie
Salute - https://t.me/CDCPiuSalute
Video - https://t.me/comedonchisciotte_video
CANALE UFFICIALE WHATSAPP:
Principale - ComeDonChisciotte.org
